HealthPulse@home Solutions
Covid 19 Specimen Collection Kits
HealthPulse@home Solutions are COVID-19 specimen collection kits that have been authorized by the FDA for Emergency Use. Leveraging Audere’s authorized labeling instructions, CLIA-certified laboratories can customize and rebrand HealthPulse@home Solutions to bring their own COVID-19 specimen self-collection kits to market without individually completing all of the steps required to obtain an FDA Emergency Use Authorization (EUA).
HealthPulse@home Solutions enable laboratories to offer specimen collection kits under Audere’s EUA — a faster, less expensive, hassle-free option for laboratories looking to offer COVID-19 test kits in the United States.
HealthPulse@home Offerings
HealthPulse@home
COVID-19 Specimen Collection Kit
Assay: LumiraDx RNA Star Complete
Collection Device: Swab and Tube
Rx Only: Yes
HealthPulse@home Fusion
COVID-19 Specimen Collection Kit*
Assay: Biosearch Technologies SaRS-CoV-2 Ultra-high Throughput End-Point RT-PCR Test
Collection Device: XPressCollect
Rx Only: Yes
What to Expect from HealthPulse@home Solutions
Simplicity
After completing our customer intake form, we will validate your eligibility for HealthPulse@home and send you a license agreement via email.
Cost Savings
HealthPulse@home services cost a fraction of what it would cost you to independently design and validate your own solution, which can amount to $100,000 or more.
Flexibility
Our authorized labeling instructions provide for custom branding, registration, and accessioning processes which require no additional usability studies.
Guidance
Our services include guidance on how to complete the steps necessary to get to market. Once all of the requirements are met, we provide you with an approval letter to get started.
Designing a COVID-19 specimen collection kit is typically time-consuming and expensive.
With us, it’s simple.
It usually requires months of work and can cost upwards of $100,000 to complete the processes and procedures required to obtain an FDA EUA. We eliminate more than half of these steps, while charging only a fraction of the cost.
Requirements to design a new solution
| Without HealthPulse@home | With HealthPulse@home | |
|---|---|---|
| FDA EUA Filing & Ongoing FDA Relationship Management | Responsibility of laboratory | ✓ Covered by HealthPulse |
| Usability Study Design, Development & Management | Responsibility of laboratory | ✓ Covered by HealthPulse |
| IFU Content Design | Responsibility of laboratory | ✓ IFU provided by HealthPulse |
| Kit Box Design & Manufacturing | Responsibility of laboratory | ✓ Guidance provided by HealthPulse |
| Kit Components Fulfillment | Responsibility of laboratory | ✓ Guidance provided by HealthPulse |
Use Cases for Unsupervised Self-collection
Community testing (drive-thru, walk-in)
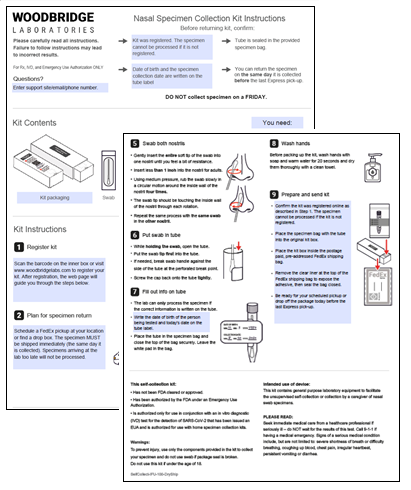
A patient arrives at a community testing center and receives a test kit containing self-collection testing supplies, instructions for use, and a patient registration/lab requisition form. The patient fills out the form and follows the instructions indicating how to collect, package, and return a nasal sample. The patient delivers the completed test kit to a site coordinator, drops it off in an approved location, or ships it back to the lab. The patient is contacted with their results after their sample is processed.
Contact tracing
A patient is identified as having potential exposure to COVID-19 and directed to order a test kit online, which is shipped directly to them. The patient receives the test kit and follows the instructions indicating how to collect and package a nasal sample. The patient is guided to schedule a pick-up from their home location or drop off the completed test kit at a local drop-box. The sample is shipped to the lab for processing and the patient is contacted with their results.
Onsite testing (office, school)
A patient arrives at their office or school and receives a test kit containing self-collection testing supplies, instructions for use, and a patient registration/lab requisition form. The patient fills out the form and follows the instructions indicating how to collect and package a nasal sample. The patient delivers the completed test kit to a site coordinator, drops it off in an approved location, or ships it back to the lab. The sample is shipped to the lab for processing and the patient is contacted with their results.
At-home test ordered online
A patient or clinician orders a test kit online, which is shipped directly to the patient. The patient receives the test kit and follows the instructions indicating how to collect and package a nasal sample. The patient is guided to schedule a pick-up from their home location or drop off the completed test kit at a local drop-box. The sample is shipped to the lab for processing and the patient is contacted with their results.
HealthPulse@home Partners
We partner with organizations that share in our mission to increase access to healthcare and improve global health. Our partners include non-profit and for-profit healthcare organizations, technology vendors, public health agencies, and government entities.
Is HealthPulse@home right for your organization?
If you are a CLIA-certified laboratory looking to quickly offer COVID-19 sample collection kits, HealthPulse@home could be right for you.
Frequently Asked Questions
Have HealthPulse@home Solutions been authorized by the FDA?
HealthPulse@home Solutions have not been FDA cleared or approved but have been authorized for emergency use by the FDA under an EUA.
HealthPulse@home Solutions have been authorized for the collection and maintenance of anterior nasal swab specimens as an aid in detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
The emergency use of HealthPulse@home Solutions is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of medical devices under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Are customers of Healthpulse@home Solutions able to define their own process for ordering and results delivery?
Customers of HealthPulse@home Solutions may define their own eligibility and ordering process which complies with the Indications for Use defined in the EUA for the in vitro diagnostic test assay. Customers of HealthPulse@home Solutions may also define their own process for results delivery to patients.
Does Audere provide test materials and kits for customers of HealthPulse@home Solutions?
Audere can provide guidance and recommended vendors for kit packaging, kit ordering, and accessioning.
HealthPulse@home Solutions will be identified as being manufactured for Audere. The lab may use the HealthPulse@home brand, but alternatively, can brand the kit as desired.
Does using this solution require the laboratory to obtain its own EUA?
No. Customers of HealthPulse@home Solutions will operate and offer their test kits under Audere’s EUA.
What is the turnaround time for contracting?
A team member will respond within 48 hours of contacting us to provide details of the additional requirements needed. Depending on the customer’s turnaround time to review the contract, an agreement could be signed within the same week.
What are the requirements to operate under Audere’s EUA?
Customers of HealthPulse@home Solutions must be a CLIA-certified laboratory to be considered eligible for HealthPulse@home Solutions. Additionally, customers of HealthPulse@home Solutions will need to submit a set of assets to Audere, with the option of contracting directly with an Audere vetted kit manufacturer, to provide this information on behalf of the lab. The assets include:
Instructions for use created using the HealthPulse@home authorized labeling instructions
Images of specimen collection kit box and components, including ship-back box (if applicable) created using the HealthPulse@home authorized labeling instructions
Description of kit manufacturing, ordering, and fulfillment practices
Description of lab accessioning procedures to ensure proper kit handling and unique kit traceability
Customers of HealthPulse@home Solutions will also need to provide a summary of the results of self-collection using a HealthPulse specimen collection kit.
Who can use HealthPulse@home Solutions?
HealthPulse@home Solutions are for use by any individual aged 16 years and older (self-collected), or 2 years and older (collected with adult assistance), including individuals without symptoms or other reasons to suspect COVID-19, for collection of anterior nares (nasal) swab specimens at home or in a healthcare setting.
How is adverse event reporting handled?
Each occurrence of an adverse event will be reported to Audere by the customer of HealthPulse@home Solutions. Medical Device Reports will be filed with the FDA as required. Audere will report such events to the FDA, in addition to the customer of HealthPulse@home Solution’s Solution’s own reporting to the FDA within five (5) days of becoming aware of the event.
What assays are supported through this service?
HealthPulse@home Solutions currently support:
LumiraDX SARS-CoV-2 RNA STAR Complete
Biosearch Technologies SaRS-CoV-2 Ultra-high Throughput End-Point RT-PCR Test